EPA Releases Draft Guidance for Pesticide Registrants on Notifications, Non-notifications, and Minor Formulation Amendments
On September 6, 2017, the U.S. Environmental Protection Agency (EPA) published a notice in the Federal Register announcing the availability of and seeking public comment on draft guidance, Pesticide Registration Notice (PR Notice) 2017-XX: Notifications, Non-notifications and Minor Formulation Amendments. EPA states it is issuing this notice to “align the notification program with the requirements of the Food Quality Protection Act (FQPA) and [the Pesticide Registration Improvement Act (PRIA)] and to clarify the processes for accepting minor, low risk registration amendments to be accomplished through notification, non-notification or as accelerated amendments.” EPA is requesting comments, and specifically information on projected cost implications of this draft updated guidance.
PR Notices are issued by the Office of Pesticide Programs (OPP). EPA states that PR Notice 2017-XX will update and clarify “the scope of changes accepted by notification, non-notification and minor formulation amendments for all pesticide products, and supersedes both PR Notices 95-2 and 98-10 in their entirety.” The PR Notice lists the changes from PRN 98-10 in a table. Those changes include:
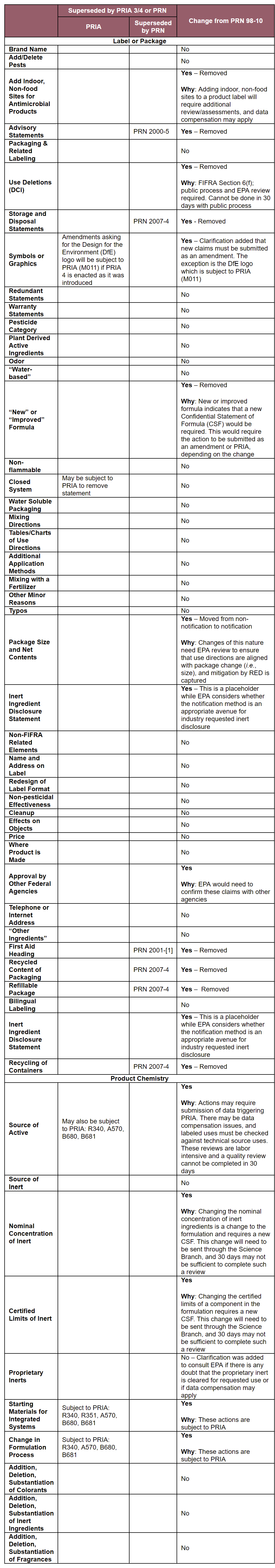
In addition to the changes listed on the table, modifications to PR Notice 98-10 consist of the following:
Notifications
- F. Product Composition: (1) Pesticide Category — Under PR Notice 98-10, the pesticide categories “disinfectant” and “sanitizer” were two pesticide categories that were allowed to be added to a label by notification. Under the proposed PR Notice, “disinfectant” and “sanitizer” were removed.
- F. Product Composition: (2) Odor — Under PR Notice 98-10, the terms “fragrance free” and “unscented” were allowed to be added to a label by a notification provided that the product is odorless or nearly odorless and contains odor-masking ingredient such as a perfume. Under the proposed PR Notice, these terms were removed.
Minor Formulation Amendments
- A. Minor Formulation Amendments: (1) Addition, deletion or substitution of one or more colorants in a formulation — Under PR Notice 98-10, if a product was intended for a use as a seed treatment or rodenticide, it would not be eligible for an accelerated review; that restriction was deleted from the proposed PR Notice.
- A. Minor Formulation Amendments: (2) Addition, deletion or substitution of one or more inert ingredients (other than colorants and fragrances) in a formulation — Under the proposed PR Notice, if a product is a dog/cat pet spot-on product or if an inert is a bittering agent or a safener, the product would not be eligible for an accelerated review.
- A. Minor Formulation Amendments: (3) Addition, deletion or substitution of one or more fragrances in a formulation — Under the proposed PR Notice, fragrances will be eligible for an accelerated review if all fragrance component ingredients are included on the Fragrance Ingredient List; individual fragrance component ingredients that exceed 0.1 percent (by weight) of the total pesticide product composition have existing approval for non-food use as an inert ingredient; and new/modified fragrances for antimicrobial products making public health claims are within the certified limits established for fragrances already approved for the product.
- Under the proposed PR Notice, products that are not eligible for accelerated review under minor formulation amendments are:
- Pet spot-on products;
- Rodenticides;
- Change to an active ingredient source;
- Change to nominal concentration of the active ingredient; or
- Addition of new or additional Confidential Statements of Formula (CSF).
EPA Procedures to Review Notifications
Under the proposed PR Notice, EPA outlines changes to the policy for processing notifications by the Registration Division (RD) and the Biopesticides and Pollution Prevention Division (BPPD), but procedures to process notifications by the Antimicrobials Division remain the same.
One item to note under the proposed notification process for RD and BPPD is that a registrant may distribute or sell a product modified by notification once EPA receives the notification but, if EPA determines that a product has been modified through notification inappropriately, EPA may initiate regulatory and/or enforcement action without first providing the registrant with an opportunity to submit an application to amend the registration.
Registrants Submitting Minor Formulation Amendments
Under the proposed PR Notice, EPA requires that registrants submit with their application for registration a cover letter listing names and dates of all EPA accepted CSFs. EPA will consider any CSFs not listed in the cover letter as superseded/no longer valid.
Comments on this PR notice are due October 6, 2017, and can be submitted online under Docket ID EPA-HQ-OPP-2016-0671.
Commentary
Registrants should review the draft PR Notice carefully, as it includes important changes. For example, the consequence for submitting a minor formulation amendment and neglecting to include a list of all current CSFs is severe. As another example, EPA signals in its proposal that proceeding to market with a product revised through the notification process may be risky if the submitter has erred in its judgment regarding what is eligible for a notification. Should the PR Notice be issued without change to this provision, submitters may wish to give close consideration to waiting until it has EPA’s written confirmation that a notification has been accepted before introducing the revised product to market. Comments on issues of concern should be considered.
